Why Is South America Becoming Ideal Ground For Covid-19 Vaccine Trials?
Last updated on November 9th, 2020 at 05:21 am
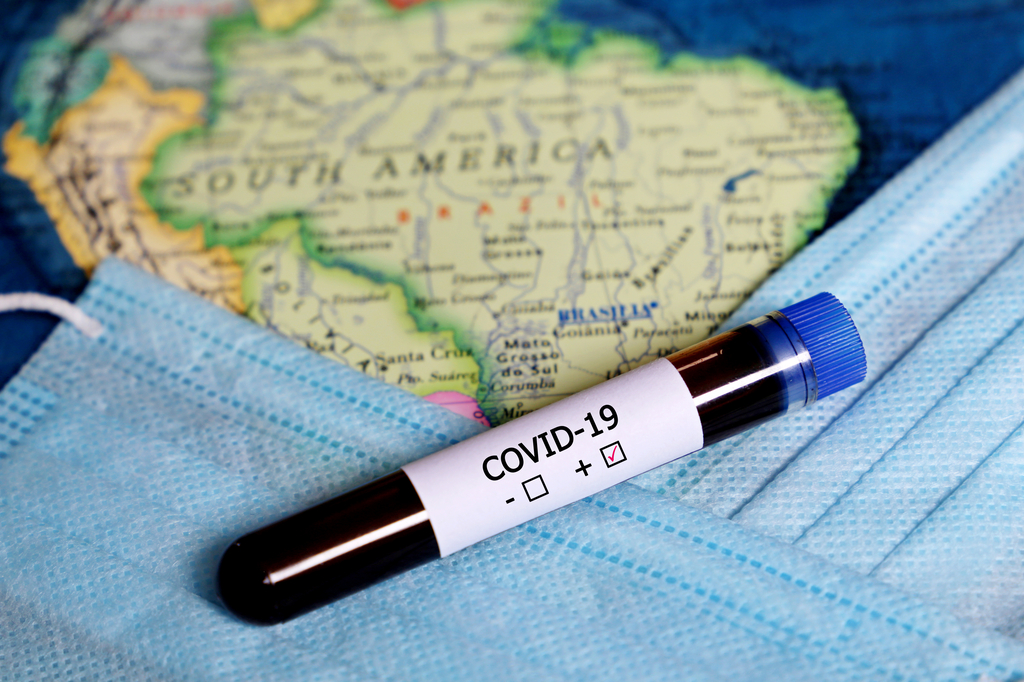
South America in Covid-19 Vaccine Trials. Imagine being chosen as the ideal place for testing and developing a vaccine because there are the highest number of cases of infections there. It is a pity but this exactly the reason that pharmaceutical companies are heading to South America.
As soon as a vaccine comes out, most residents of the smaller provinces of South America feel they will not be on the priority list for the vaccine. Most locals’ complaint is that they seem to have been forgotten and feel used for the trails.
Companies like German Biontech and Curevac are preferring to be in South only because the high local infection rates makes it for an ideal testing ground. The world continues to races to find an effective vaccine against the coronavirus.
The various administrations in Panama and Peru for example see an economic advantage in being chosen as a trial ground. Peru for example has become the first testing ground. Belavista district of Peru has reasons not to be bothered about a possible vaccine because they say that they have not even received support from their government in the time of the pandemic.
Brazil has already seen many pharmaceutical companies set up shop too. In Sao Polo for example, America giant Pfizer and Germany company Biontech are busy testing people. They are using volunteer subjects that are being administers with candidate vaccines or a placebo. None of them paid for being used as Guinee pigs.
Cepic is a Portuguese company that undertakes specialized clinical trials. According to the Director of Cepic, Cristino Zerbini, “Most of our subjects are commoners working to make ends meet. We think this vaccine will be effective.” They feel proud to be associated with these clinical trials.
Trials seem to be most aggressively underway in Brazil that anywhere else. This is again because the country has the largest number of Corona virus cases. Biontech is confident that it can receive approval for its vaccine before the end of 2020. Curevac is still going to carry out trials in Panama too. This is another country thathas high infection rates, a fact that is helping to ascertain the effectiveness of the vaccine. For Panama, this is an economic advantage over the rest of the world.
Sharing technology is also a part of the important deal between pharma
companies and countries holding such trials. But whether the common man who is participating in the trial will be let down by their respective government, or will they get a dose to protect themselves first; is something that will have to be left for time to tell.